QligFEP has been used on different targets, examples can be found in the publications below.

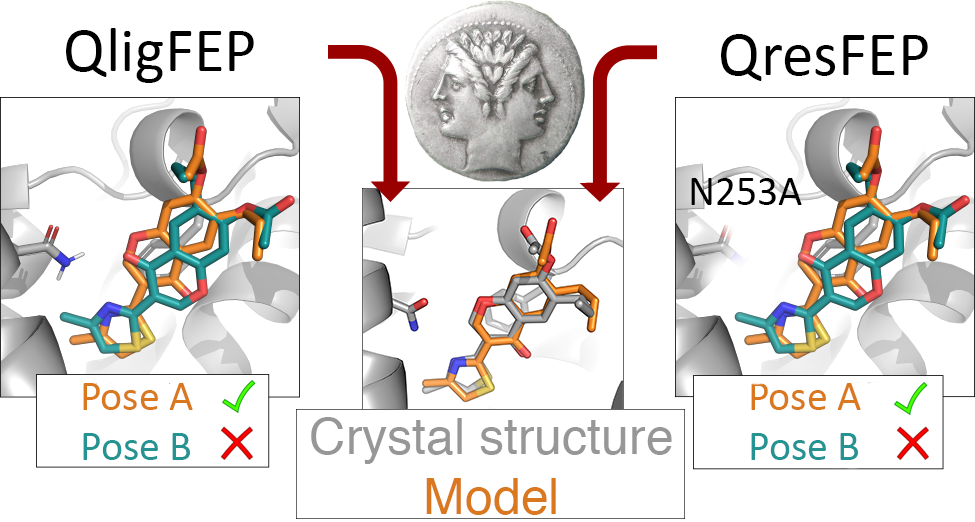
GPCR: Adenosine A2A Receptor
X‐Ray Crystallography and Free Energy Calculations Reveal the Binding Mechanism of A2A Adenosine Receptor Antagonists
W. Jespers, G. Verdon, J. Azuaje, M. Majellaro, H. Keränen, X. García‐Mera, M. Congreve, F. Deflorian, C. de Graaf, A. Zhukov, A.S. Doré, J.S. Mason, J. Åqvist, R.M. Cooke, E. Sotelo, H. Gutiérrez‐de‐Terán, Angewandte Chemie International Edition 2020, 59 (38), 16536-16543.
GPCR: Adenosine A2B Receptor
3,4-Dihydropyrimidin-2(1H)-ones as Antagonists of the Human A2B Adenosine Receptor: Optimization, Structure–Activity Relationship Studies, and Enantiospecific recognition
M. Majellaro, W. Jespers, A. Crespo, M.J. Núñez, S. Novio, J. Azuaje, R. Prieto-Díaz, C. Gioé, B. Alispahic, J. Brea, M.I. Loza, M. Freire-Garabal, C. Garcia-Santiago, C. Rodríguez-García, X. García-Mera, O. Caamaño, C. Fernandez-Masaguer, J.F. Sardina, A. Stefanachi, A. El Maatougui, A. Mallo-Abreu, J. Åqvist, H. Gutiérrez-de-Terán, E. Sotelo, Journal of Medicinal Chemistry 2021, 64 (1), 458–480.

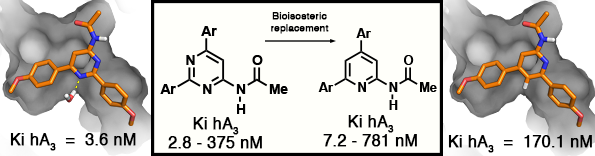
GPCR: Adenosine A3 Receptor
Effect of Nitrogen Atom Substitution in A3 Adenosine Receptor Binding: N -(4,6-
Diarylpyridin-2-yl)acetamides as Potent and Selective Antagonists
J. Azuaje, W. Jespers, V. Yaziji, A. Mallo, M. Majellaro, O. Caamaño, M.I. Loza, M.I. Cadavid, J. Brea, J. Åqvist, E. Sotelo, H. Gutiérrez-de-Terán, Journal of Medicinal Chemistry 2017, 60 (17), 7502-7511.
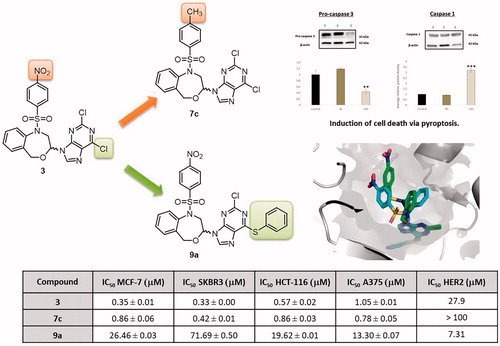
Kinase: Human epidermal growth factor receptor 2 (HER2)
Design, synthesis, HER2 inhibition and anticancer evaluation of new substituted 1,5-dihydro-4,1-benzoxazepines
O. Cruz-López, M. Ner, F. Nerín-Fonz, Y. Jiménez-Martínez, D. Araripe, J. A. Marchal, H. Boulaiz, H. Gutiérrez-de-Terán, J. M. Campos, A. Conejo-García, Journal of Enzyme Inhibition and Medicinal Chemistry 2021, 36 (1), 1553–1563.
NNMT: Nicotinamide N-Methyltransferase
Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics.
Y. Gao, M. J. van Haren, N. Buijs, P. Innocenti, Y. Zhang, D. Sartini, R. Campagna, M. Emanuelli, R. B. Parsons, W. Jespers, H. Gutiérrez-de-Terán, G. J. P. van Westen, N. I. Martin, Journal of Medicinal Chemistry 2021, 64 (17), 12938–12963.
